Cells and Ions
This is a drawing of one of the 86 billion or so neuron cells that make up your brain. If you are interested in understanding how these machines work, follow along.
Its Very Complex
Prof. Jeff Lichtman - Harvard
The complexity in the brain is by far in excess of the complexity of any other thing humans have confronted. And this is a very hard and painful pill to swallow for scientists, when you're trying to understand something that has so much complexity.
And let me give you a sense of how complex it is.
In a technique like functional magnetic resonance imaging, where the brain is divided into voxels-- little cubic points of data that's related to blood flow-- the grain of that image is about one cubic millimeter per voxel. And that still gives rise to a million voxels in a brain. So, there's a huge amount of data in fMRI images.
If we take one of those cubic millimeter voxels and ask, how much resolution do we need to see all these synapses in that voxel? We need about 2,000 terabytes of data-- 2 petabytes per cubic millimeter.
So, if we wanted to do a whole human brain, we would deal with like 2 million petabytes of data-- 2 million, million terabytes-- which is comparable to the digital content of the world.
It's an extraordinarily large number and much more than will fit on my laptop.
1.0 The Cell
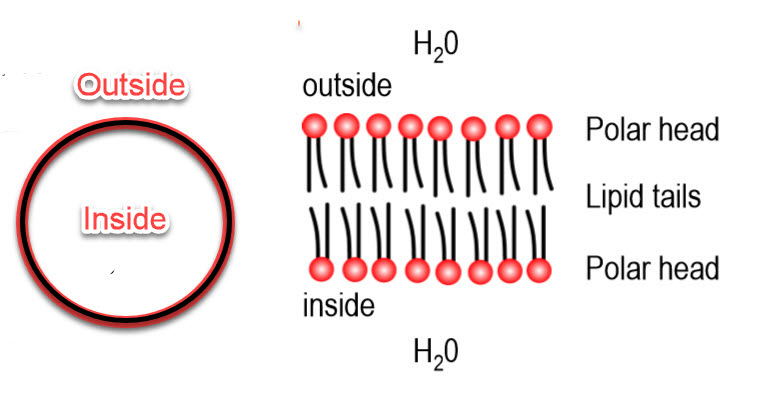
The cell is the fundamental unit of organization of the brain, and its key defining feature is the cell membrane. It separates the inside of a cell from the outside of a cell. When the cell membrane evolved some three billion years ago, its primary advantage was to offer a stable intracellular environment for chemical reactions to take place that would not be affected by changes in the extracellular environment. The task of environmental protection was chosen by evolution to fall to lipids and a lipid by-layer for the cell membrane - this is how that works:
Lipids are a class of organic compounds that are fatty acids or their derivatives and are insoluble in water but soluble in organic solvents. They include many natural oils, waxes, and steroids. The cell membrane is made of phospholipids with the chemical signature shown below. Each of these consists of a phosphate-containing head, and twin long hydrocarbon chains.
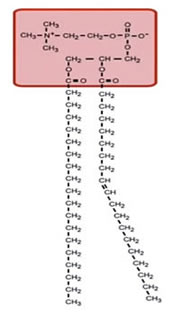
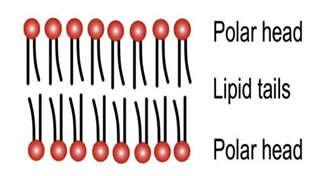
The chains are lipophilic and non-polar, and therefore they are hydrophobic; they don't like to interact with water. The phosphate heads are highly polar and carry a strong negative charge. They want to interact with water; that is, they are hydrophilic.
Lipid Bilayer
If you were to collect a few million lipids and drop them in water, they amazingly spontaneously form a planar lipid bilayer membrane.
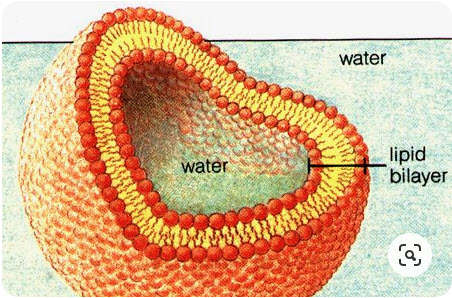
The lipid membrane is:
- Highly permeable to gases and small uncharged molecules
- Limited permeability to water
- Impermeable to ions and charged molecules
Ions
Ions are the most important element in the operation of the brain. They are charged, and this charge forms the basis of electrical signaling in the nervous system. Because the membrane is impervious to ions means that there can be different concentrations of ions on the two sides. For example, in a typical cell, there is a high concentration of positively-charged potassium ions on the inside, and on the outside (the extracellular solution), there is a high concentration of positively-charged sodium ions and a high concentration of negatively-charged chloride ions.
So the extracellular solution is made of Sodium chloride or salt - simple kitchen salt.
The membrane is very thin, about five nanometers, allowing ions on one side of the membrane to react to the charge of ions on the other. This causes the membrane to act like a capacitor. It's a dielectric with strong fields across the lipid membrane.
This is the membrane potential.
Movement of Charge
By measurement, the differential voltage between the inside and outside of the cell is about -100 mV. The outside, by convention, is always at 0 volts. In addition, the capacitance of the membrane is about one microfarad per square centimeter. With this in mind, we can calculate how much positive change must move from the inside to the outside to give the inside that -100 mV change.
We know that the area of a small circular cell has a radius of around 10 micrometers, which puts the capacitance at about ten picofarads. We also know that the voltage across a capacitor V is equal to the charge of the capacitor Q in coulombs divided by the capacitance C.
Where:
- C the capacitance of the cell ( 10 X 10E-12 farad) and
- Q charge in coulombs
- V the voltage across the cell (0.1 Volt)
Therefore:
Q = 1 X 10E -12 coulomb or 1 picocoulomb (pC)
The inside of the membrane contains a large number of positively charged potassium ions. How many of these would be required to move to make that 100 mVolt of charge?
Each ion carries a single charge, and that charge is given by the elementary charge of an electron or 1.6 X 10E -19 coulomb:
Therefore 1 X 10E-12 Coulomb divided by 1.6 X 10E -19 ~ six million ions that need to move from the inside to the outside to produce 100 mV differential. That seems like a large number, but there are about 1 X 10E 11 potassium ions inside a typical cell so only a small number of ions available need to be moved to make this significant change.
So how do these ions move across the membrane? The answer is Ion Channels.
Ion Channels
There are several types of Ion channels. The ones we are dealing with here are the 'Always open' and 'Voltage-gated'
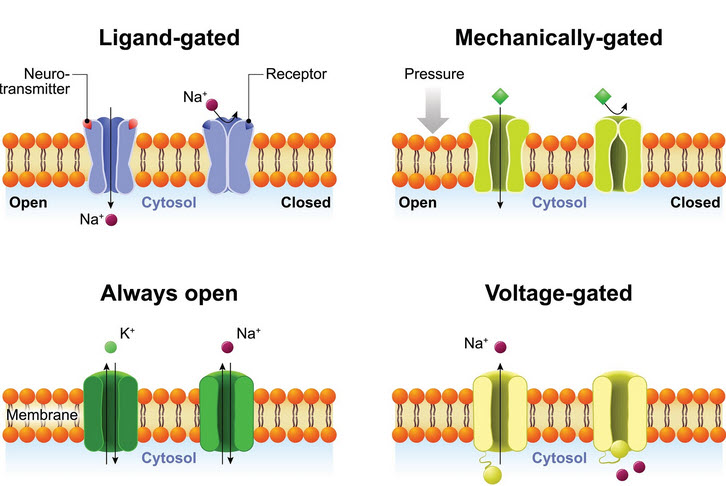
The phospholipid bilayer isn't itself permeable to ions. So, how do ions move across the cell membrane? How does the membrane potential change? This is done by ion channels that traverse the membrane. They have pores in the middle that allows Potassium, Sodium, and Chloride ions to move through.
This video depicts the operation of ion channels as they move Potassium ions and their positive charge from one side of the membrane to the other.
The video shows the operation of only Potassium channels but other channels exist as well:
- Potassium - K+
- Sodium - Na+ and
- Chloride - Ci-
The operation of the complete set of channels is complex and but well understood. Thankfully we do not have to have a complete understanding of these molecules to move forward.
Membrane Potential
We know that the membrane acts as a capacitor and that ion channels act as conductors for specific ions. We can now see how the cell properties of electrochemical diffusion determine the membrane potential. Consider a single piece of the membrane with a potassium-selective ion channel, as shown below. There are two main forces at work, the concentration gradient and the electrical gradient.
Concentration Gradient
If the potassium concentration is high inside the cell compared to the outside potassium will flow down the gradient to the outside. Since there is a large amount of potassium inside (shown by the large K+) as compared with the outside, a concentration gradient exists that allows diffusion across the membrane.
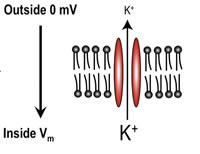
Electrical Force
There is a force applied to any charged ion that depends upon the spatial gradient of the electric field. This is one of the significant contributors of ionic movement and is shown by the equation below.
If we have a negative potential on the inside, then potassium ions will be attracted to the inside down the electric-field gradient.
Balance
The concentration gradient and the electrical gradient oppose each other and precisely balance each other out at one specific potential - about -90 mV. This 'reversal potential this the point at which there's no net flux of ions.
The Nernst equilibrium equation for potassium ions is used to calculate this value.
Where:
- Ek is the reversal potential for potassium
- Ko outside = 5 mM
- Ki inside = 150 mM
- R is Avogadro's gas constant
- T is Temperature in kelvins
- Z is the charge on the ion (for Potassium +1, calcium +2, chloride -1)
- F is the electrical force
RT over zF multiplies the logarithm of the ratio of the outside ionic concentration to the inside. This produces ~-90 mV reversal potential for Potassium.
Although Potassium is a very important ion, it is not the only one. Plugging in values for different concentrations provides the equilibrium potential for some of the others.
| Ion | Inside | Outside | Name | E ion |
|---|---|---|---|---|
| K+ | 150 mm | 4 mM | Potassium | -97 mV |
| Na+ | 12 mM | 145 mM | Sodium | +67 mV |
| Ci- | 5 mM | 120 mM | Chloride | -85 mV |
| Ca2+ | 100 nM | 1 mM | Calcium | +123 MV |
Since each cell has a mixture of potassium, sodium and chloride that effect the membrane potential the result is a equilibrium potential of about -60 mV. This is called the resting potential - VRest.
The movement of ions from one neuron to another is the subject of Synaptic Transmission and is fully covered in all textbooks on neurology. It is fascinating but will not be repeated here except to clarify the terms EPSP and IPSP.
EPSP and IPSP
If the open channels are permeable to Na, the net effect will be to depolarize the post-synaptic cell from the resting membrane potential. Because it tends to bring the membrane potential toward the firing threshold for action potentials, it is said to be excitatory.
A transient post synaptic membrane depolarization caused by the synaptic release of neurotransmitter is called an Excitatory Post-Synaptic Potential (EPSP).
If the transmitter-gated channels are permeable to Cl, the net effect will be to hyperpolarize the post-synaptic cell from the resting membrane potential because the chloride equilibrium potential is negative. Because it tends to bring the membrane potential away from the threshold for generating action potentials, this effect is said to be inhibitory.
A transient hyper polarization of the post-synaptic membrane potential caused by the synaptic release of neurotransmitter is called an Inhibitory Post-Synaptic Potential(IPSP)
More on the use and simulation of EPSP and IPSP is in section on Dendrites.
